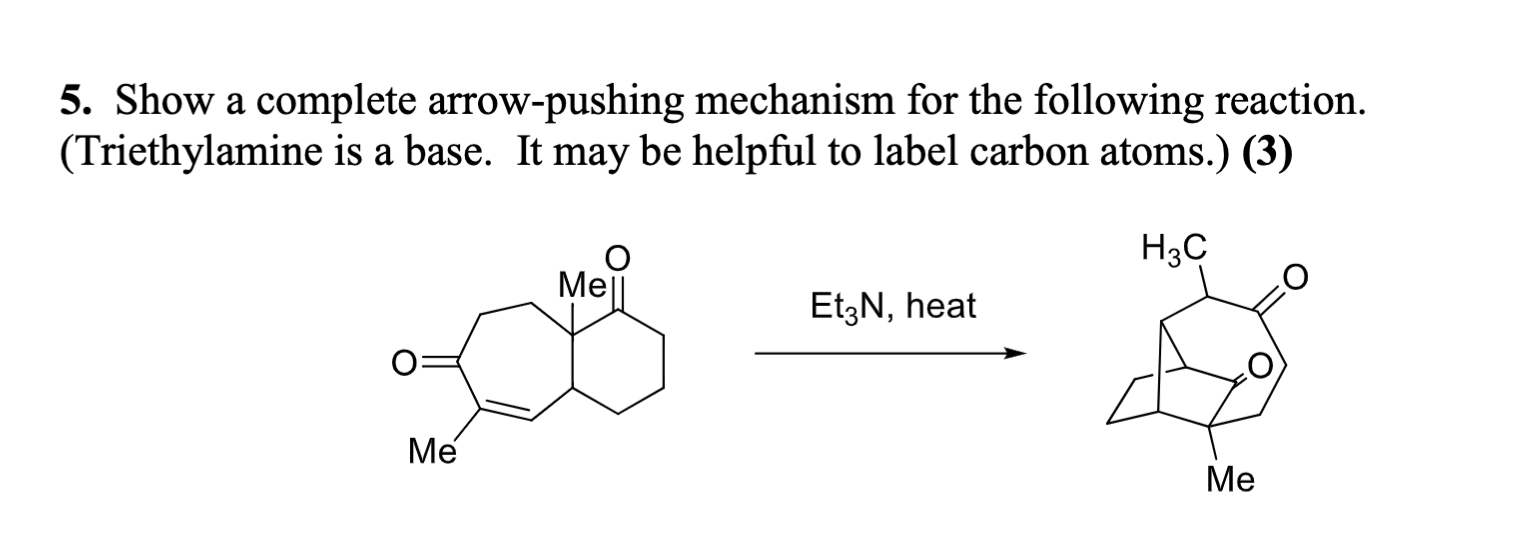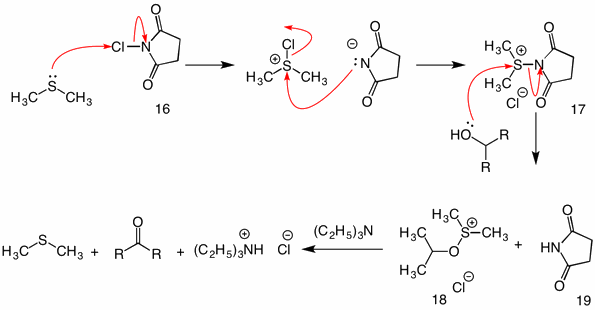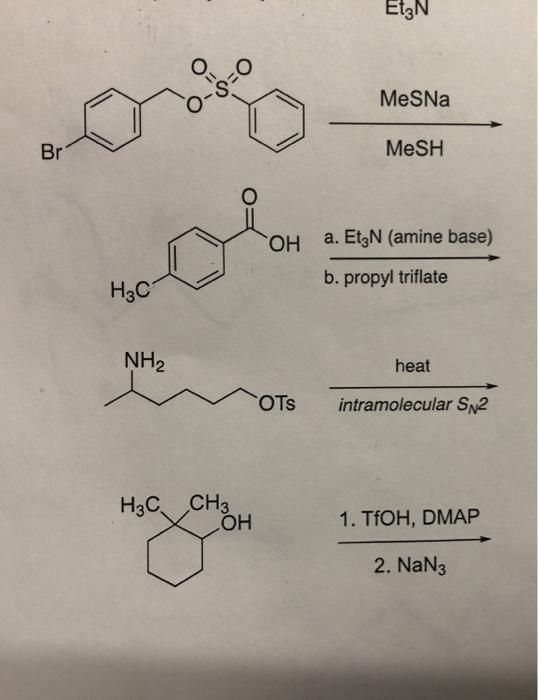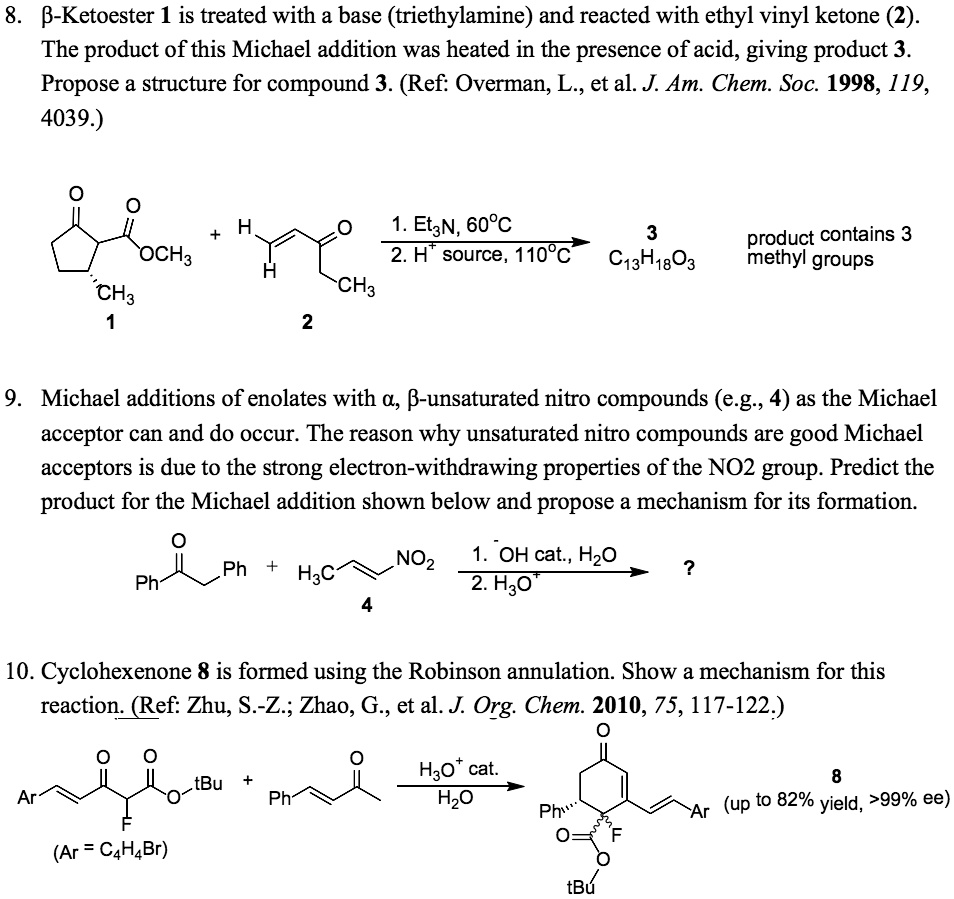
SOLVED: 8. B-Ketoester 1 is treated with a base (triethylamine) and reacted with ethyl vinyl ketone (2). The product of this Michael addition was heated in the presence of acid, giving product

CuCl/Et3N-Catalyzed Synthesis of Indanone-Fused 2-Methylene Pyrrolidines from Enynals and Propargylamines | Organic Letters

Complete the below acid-base reaction and name the salt formed. (a) Et3N+HCl gives to (b) C5H11NH+CH3COOH gives to | Homework.Study.com

Mechanisms for interaction between acetic acid and triethylamine, (a)... | Download Scientific Diagram

entry 5). The use of other bases such as Et3N, DIPEA, or NH4OAc did not... | Download Scientific Diagram

organic chemistry - Why the formation of a fog is observed when triethylamine is added? - Chemistry Stack Exchange

Triethylamine Hydroiodide as a Bifunctional Catalyst for the Solvent‐Free Synthesis of 2‐Oxazolidinones - Nishiyori - 2020 - European Journal of Organic Chemistry - Wiley Online Library