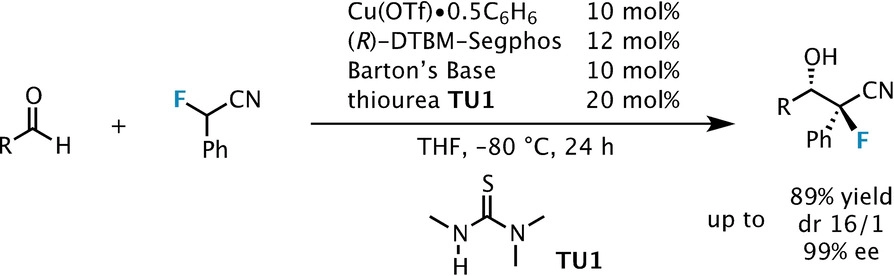
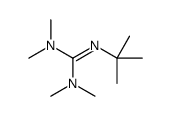
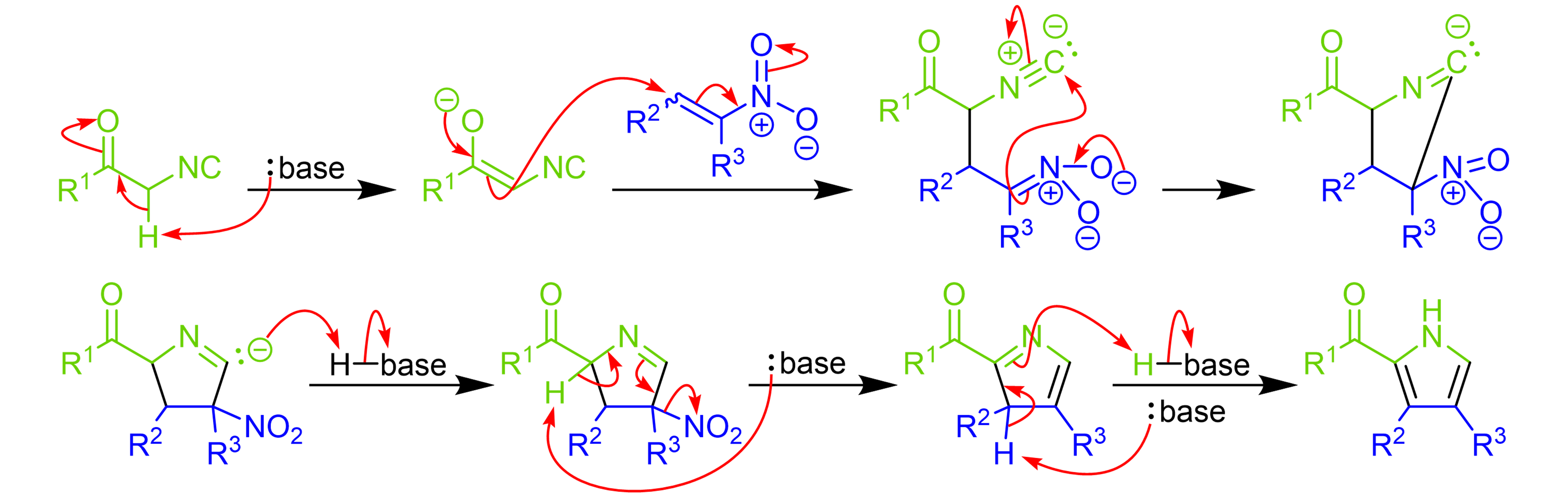
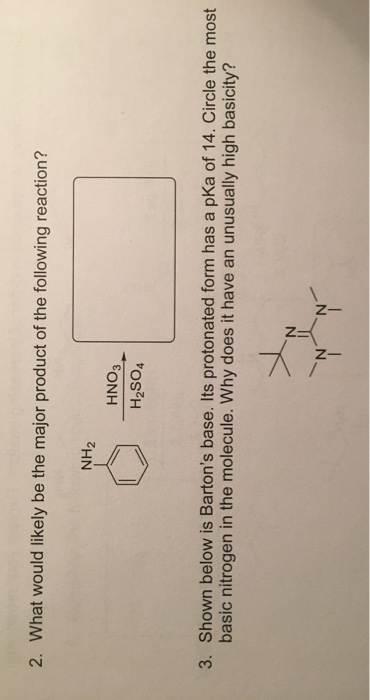
Synthesis of Highly Oxygenated Dinaphthyl Ethers via SNAr Reactions Promoted by Barton's Base | Organic Letters
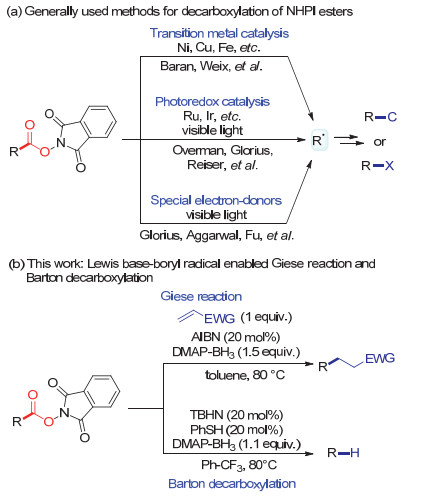
Lewis Base-Boryl Radical Enabled Giese Reaction and Barton Decarboxylation of N-Hydroxyphthalimide (NHPI) Esters

Construction of Chiral 2,3‐Allenols through a Copper(I)‐Catalyzed Asymmetric Direct Alkynylogous Aldol Reaction - Zhong - 2020 - Angewandte Chemie International Edition - Wiley Online Library

Proposed catalytic cycle and TS of the present vinylogous addition of... | Download Scientific Diagram

Copper(I)‐Catalyzed Asymmetric Vinylogous Aldol‐Type Reaction of Allylazaarenes - Wang - 2021 - Angewandte Chemie International Edition - Wiley Online Library

The 140th Annual Meeting of the Pharmaceutical Society of Japan (Kyoto)/Catalytic Asymmetric Synthesis of Chromanone Lactones Using Environmentally Friendly Vinylogous Michael Reactions

Direct Catalytic Asymmetric Mannich-Type Reaction en Route to α‑Hydroxy-β-amino Acid Derivatives - ScienceDirect
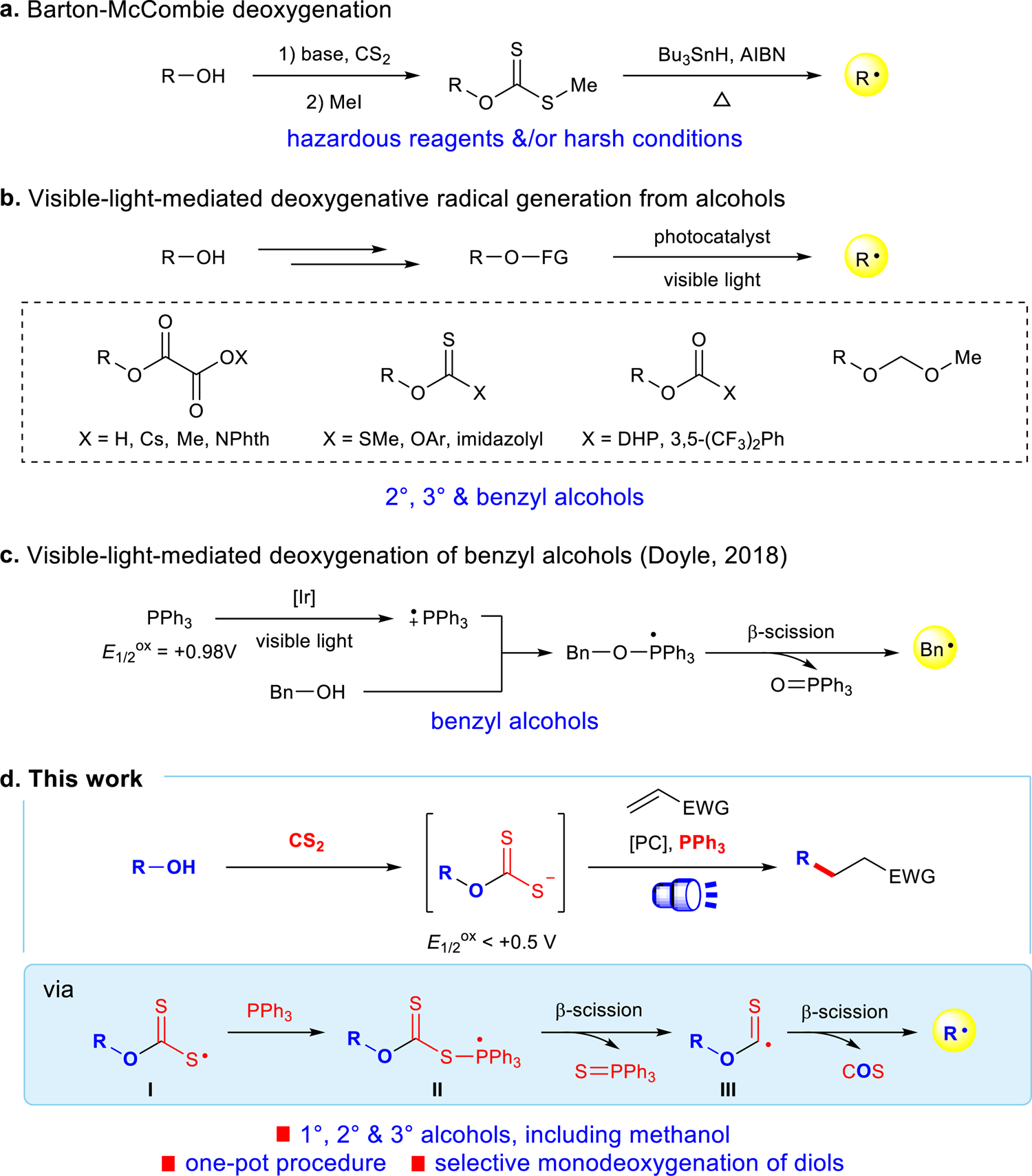
Selective deoxygenative alkylation of alcohols via photocatalytic domino radical fragmentations | Nature Communications

Rapid Synthesis of Chiral 1,2‐Bisphosphine Derivatives through Copper(I)‐Catalyzed Asymmetric Conjugate Hydrophosphination - Yue - 2020 - Angewandte Chemie International Edition - Wiley Online Library

Brønsted Base‐Catalyzed Direct 1,6‐Conjugate Addition of Butenolide to p‐Quinone Methides | GDCh.app